A note on using Google Slides
It is worth pointing out that although I rely heavily on Google Slides in my classroom, they do not carry out the most important of teacher skills: clear, concise explanations and careful questioning to gauge individual understanding. I use these slides to provide a clear structure to my learning sequences, provide the start points for explanations, and include ready made questions for retrieval practice, diagnosis of understanding and practice. I have found this to greatly improve my efficiency (particularly in reducing preparation time) and students seem to value the slides as a resources for review as well as being helpful for missed classes.
What's in the slides?
Please note that the resources here are a culmination of 10 years of IB teaching. I have tried to avoid any copyright infringement but inevitably the odd activity/resource/past-paper question will have slipped through the net. Please let me know if you spot any issues through the Contact form on the homepage.
The slides in each deck are colour-coded as follows:
The slides in each deck are colour-coded as follows:
BLUE: Intro, prior knowledge check and SL/HL indicator
|
The first slide in each deck outlines the subtopic, guiding question and recommended time allowance.
|
The second slide is an opportunity to identify preexisting conceptual understandings/misconceptions.
|
These slides will identify parts of the slide deck as either 'SL and HL content' or 'AHL content'.
|
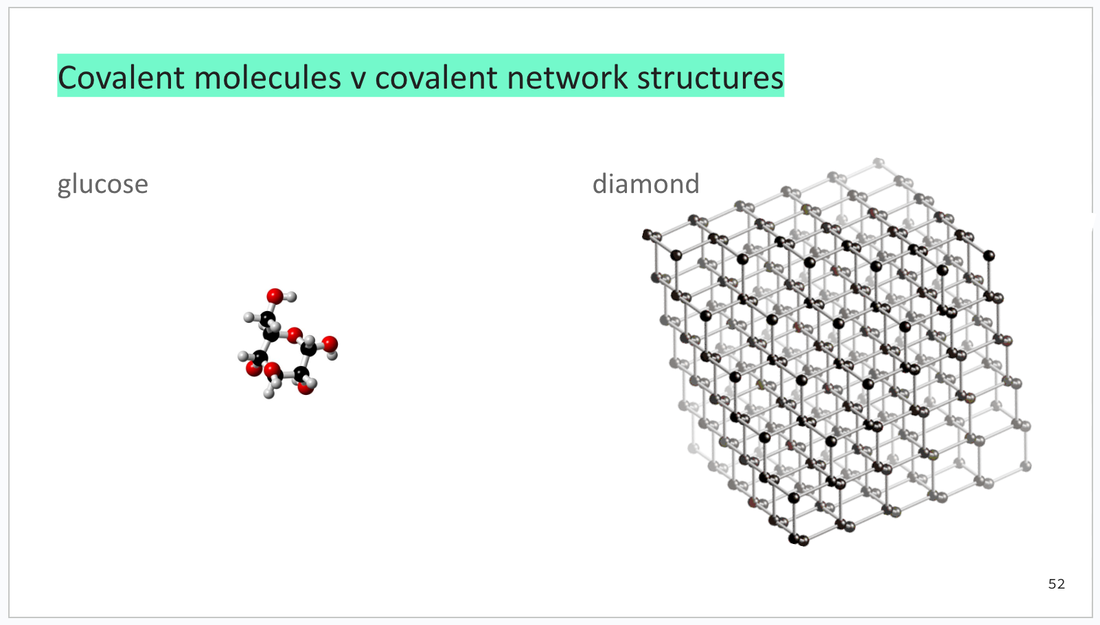
GREEN: Syllabus content
|
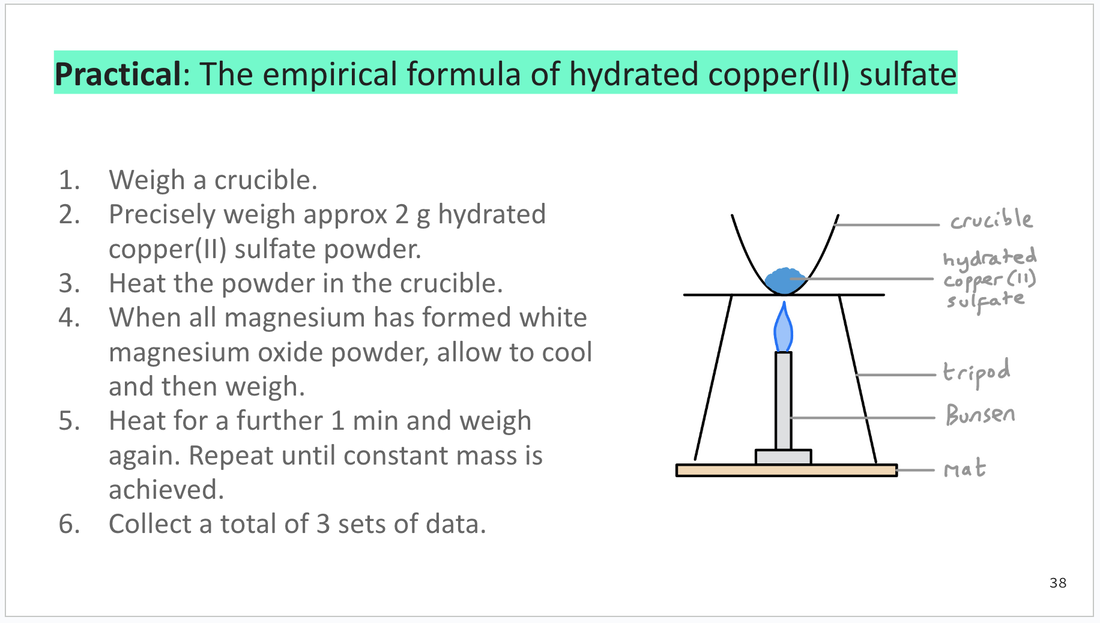
Practical work and with a simple risk assessment (info from CLEAPSS).
|
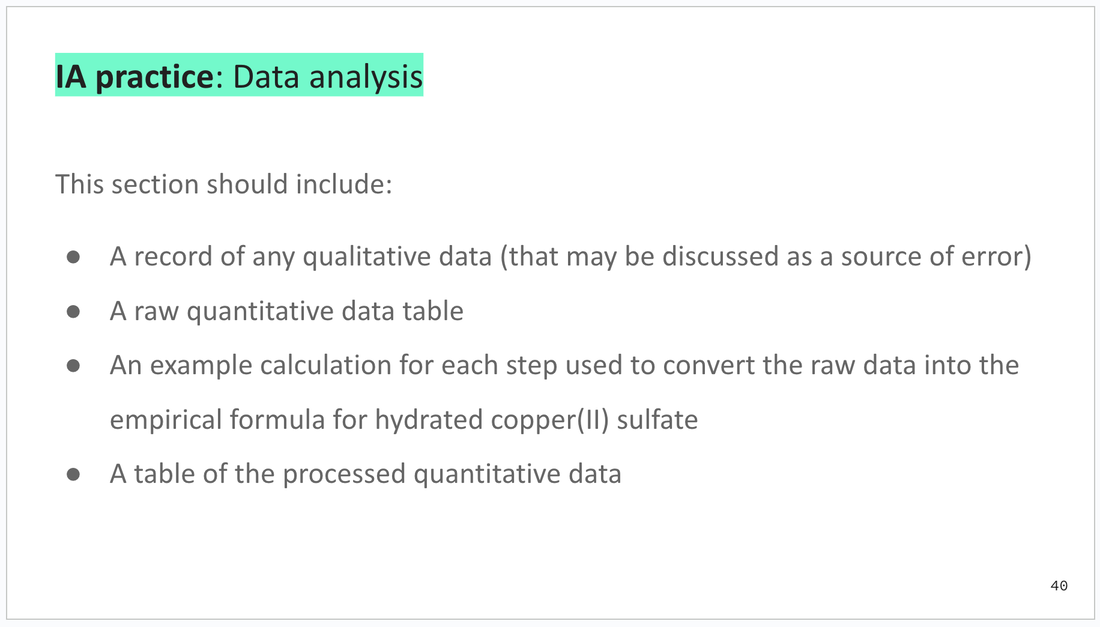
Practice related to the IA (scientific investigation) following practical work.
|
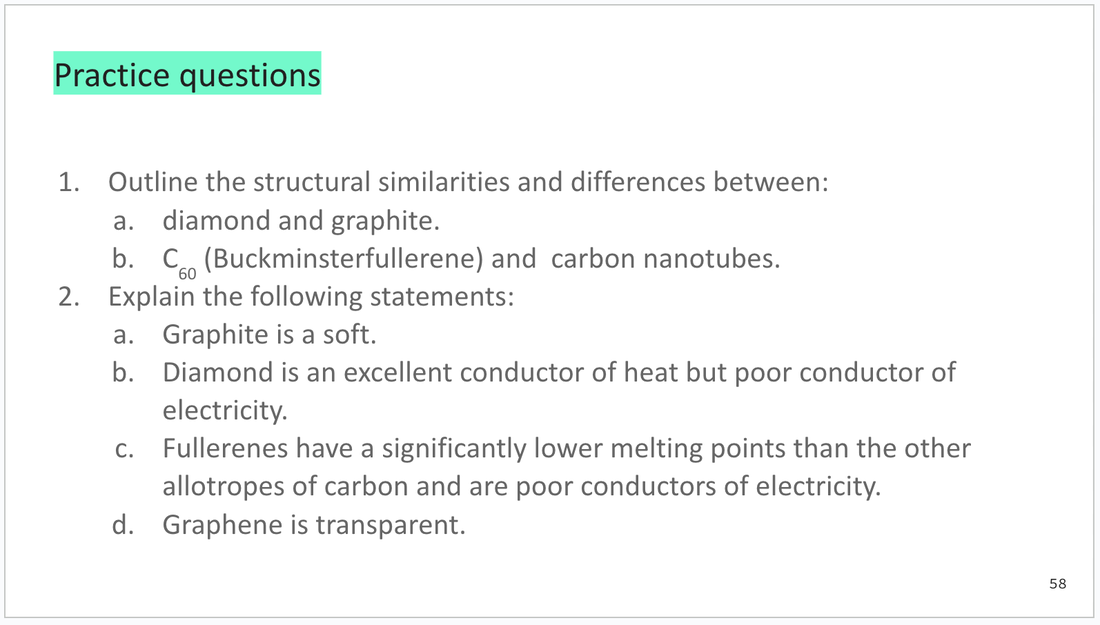
Practice questions are found at the end of each 'part'.
|
YELLOW: Guiding question, key terminology and retrieval practice
|
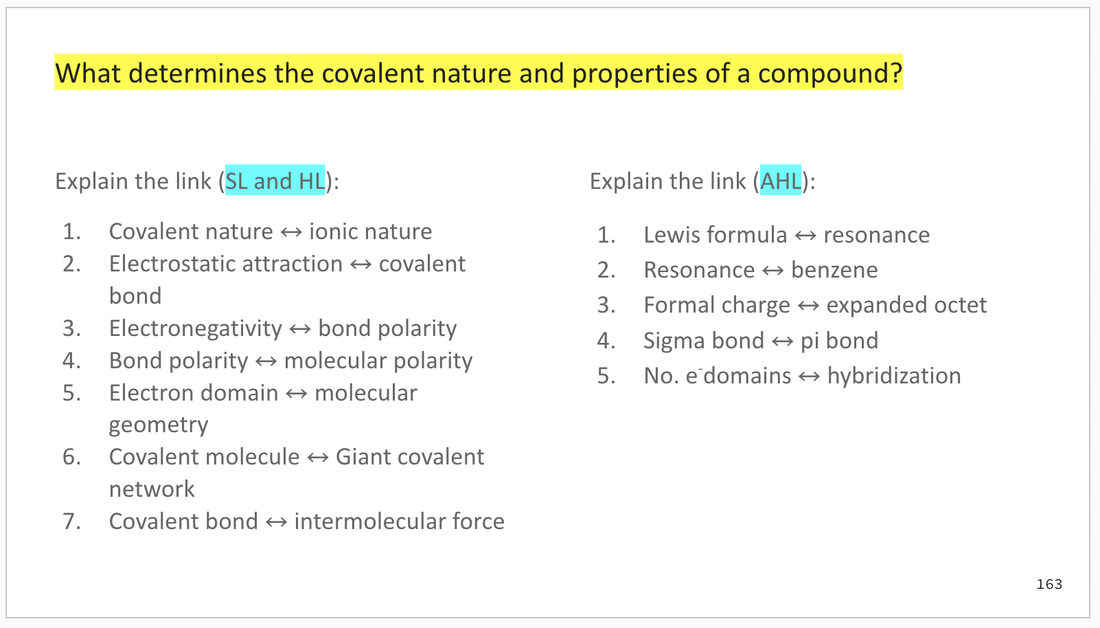
At the end of the content slides, you will find an activity that engages with the subtopic guiding question.
|
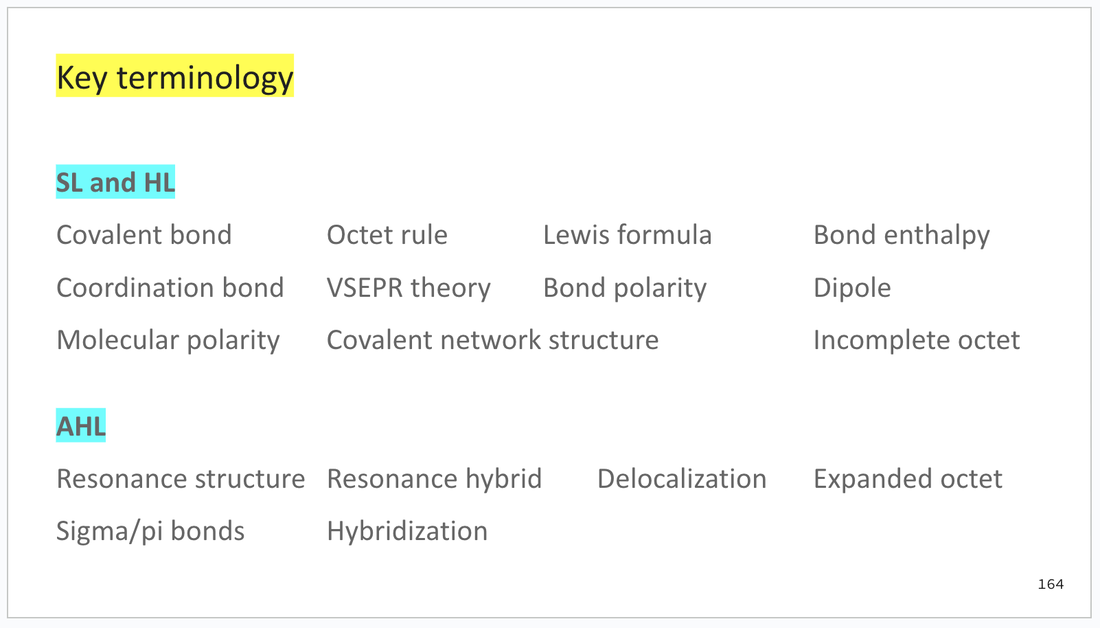
There is also a list of key terminology from the subtopic.
|
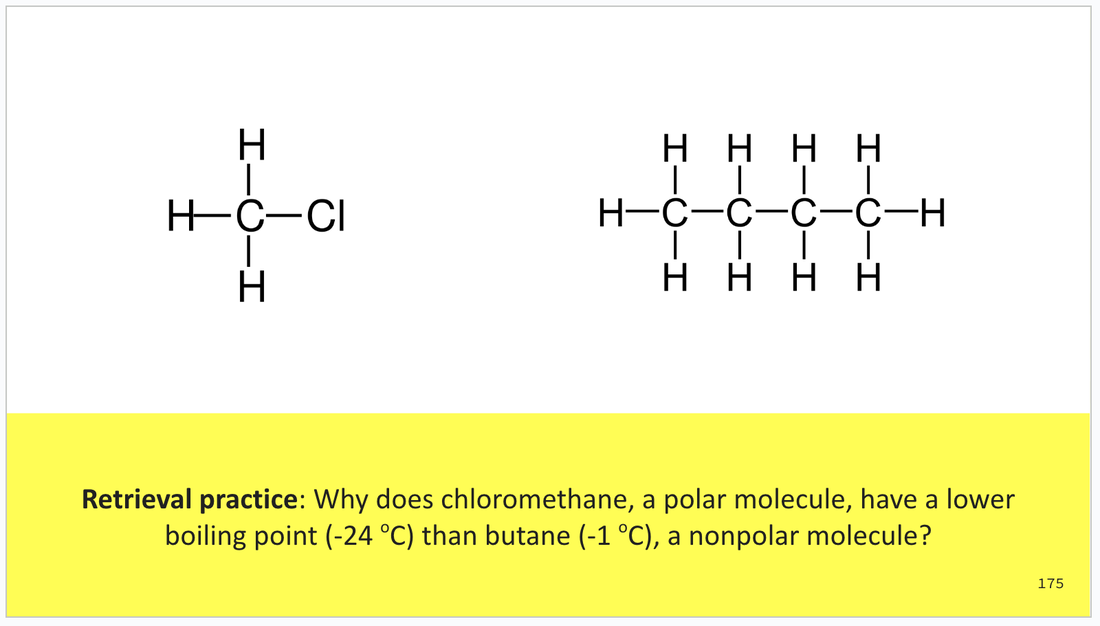
And a section with a range of retrieval practice slides to be integrated at the teacher's discretion throughout a learning sequence.
|
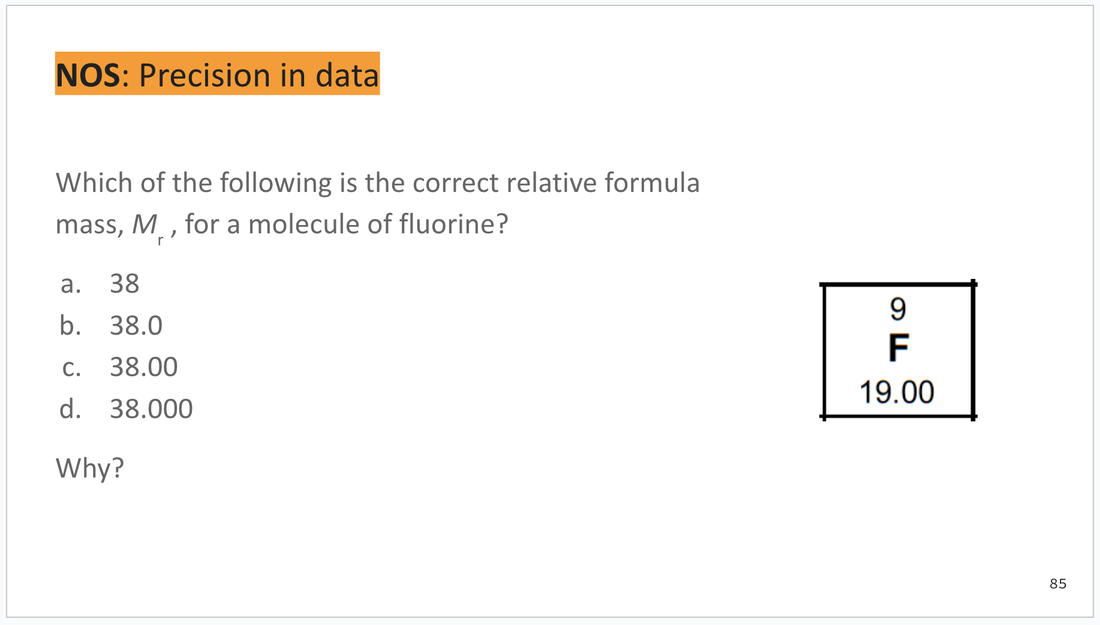
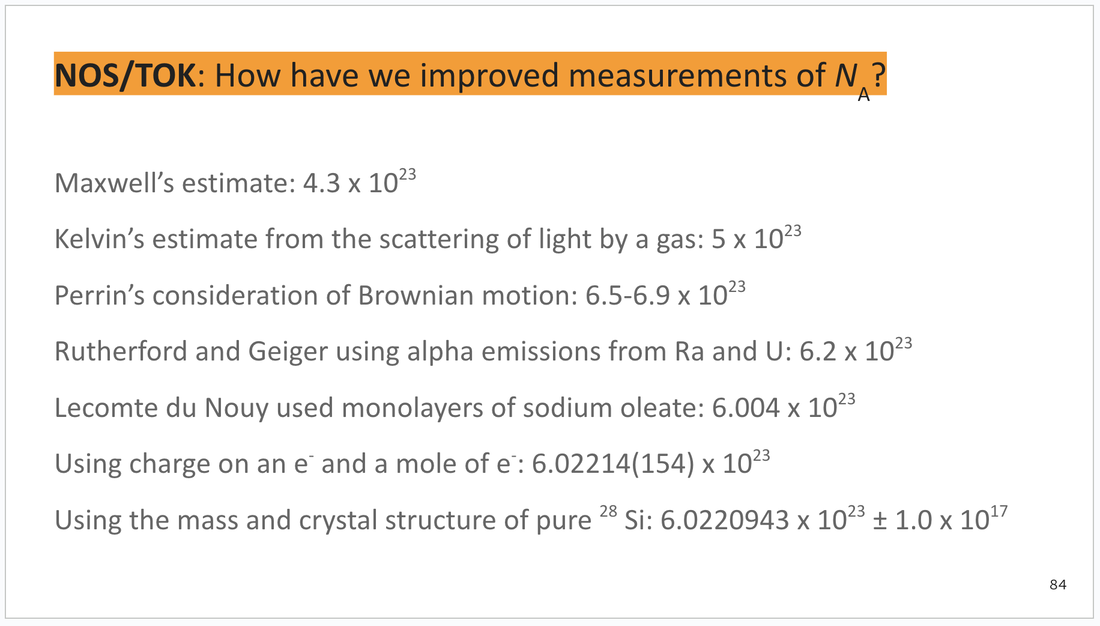
ORANGE: Nature of science (NOS) and theory of knowledge (TOK)
These slides are found at the bottom of each deck and can be integrated as you wish. I will try to include possible answers or key points for discussion in the speaker notes section below each slide.
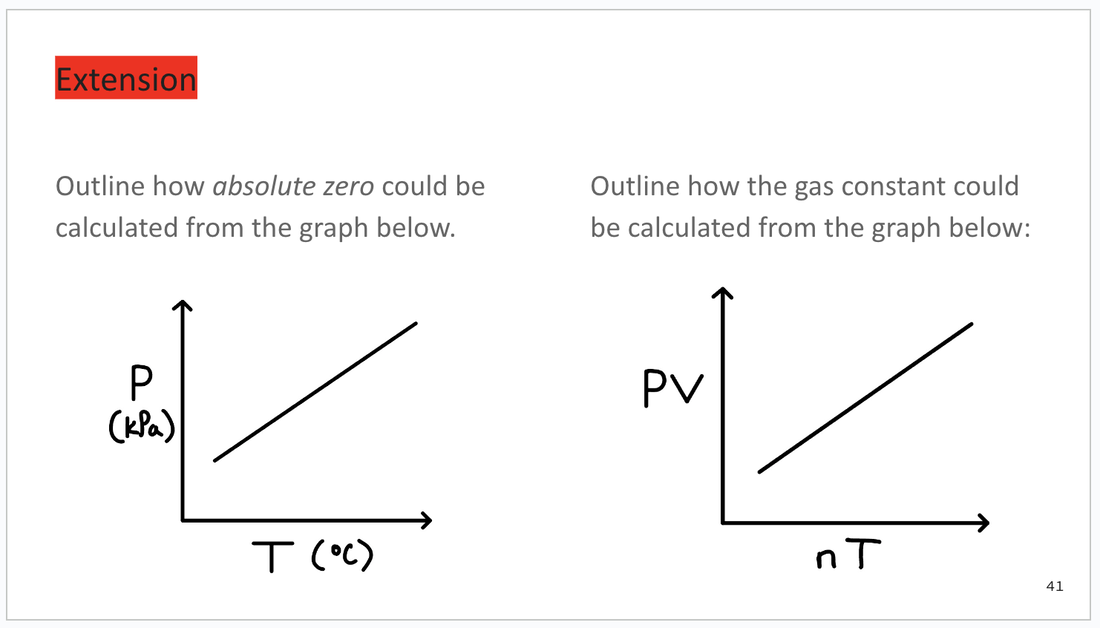
RED: Extension
At the very end of each slide deck, you will find some challenging extension questions. Again, I will try to include possible answers to these questions in the speaker notes.